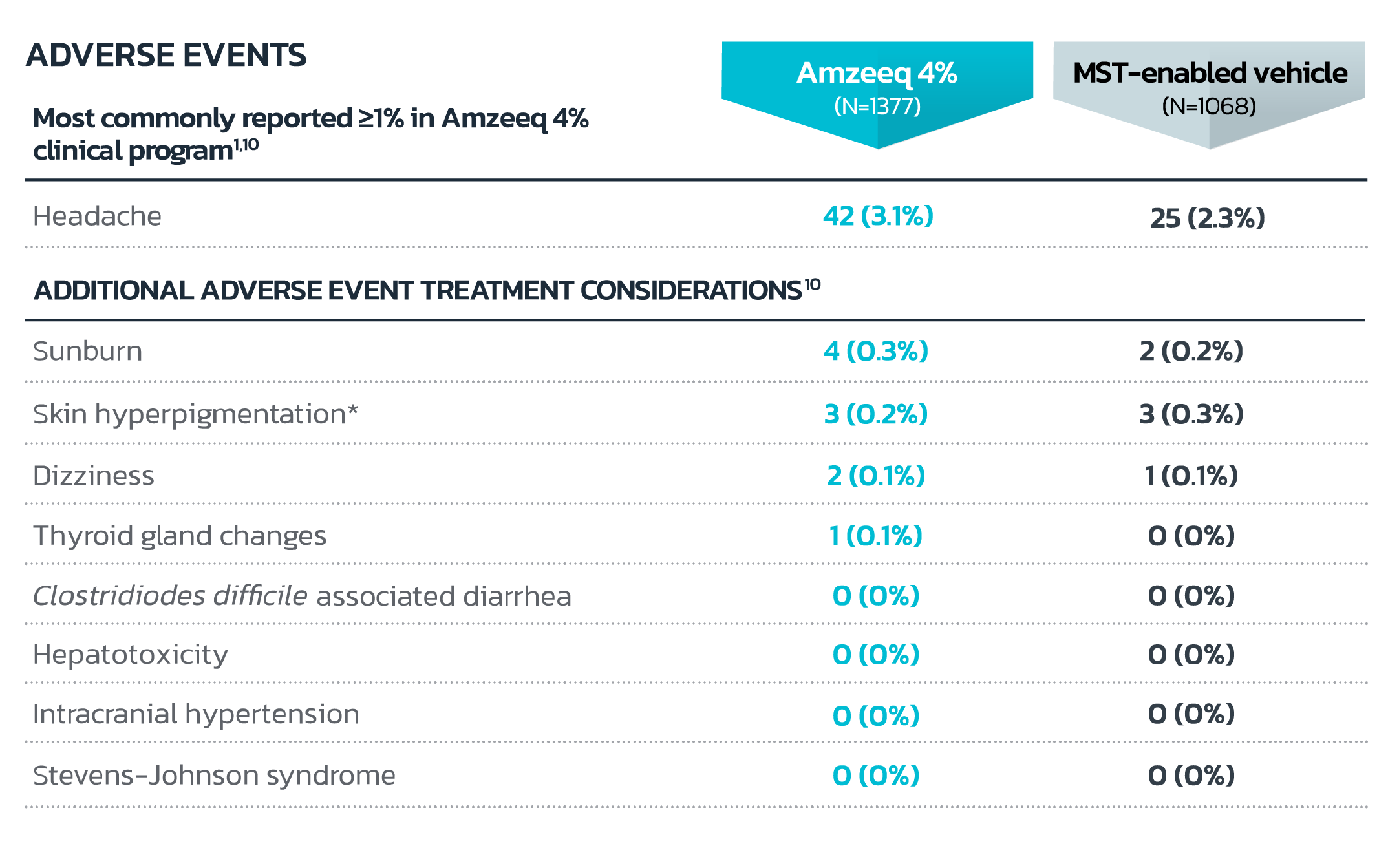
- *Skin discoloration (2 vs 3) and skin hyperpigmentation (1 vs 0) for Amzeeq vs vehicle respectively. In Amzeeq clinical trials, there were no reported cases of minocycline-induced blue-gray skin hyperpigmentation.
- Local tolerability evaluations were conducted at each study visit for 12 weeks in the clinical trial by assessment of erythema, dryness, hyperpigmentation, skin peeling and itching. Local tolerability signs and symptoms occurred in similar frequency and severity as subjects treated with the vehicle component of Amzeeq1
- In an additional 40-week, open-label, extension safety study (up to total of 52 weeks of treatment), frequency and severity of local tolerability signs and symptoms, as well as adverse events, were comparable at Week 52 to those reported at Week 121,8
Important Safety Considerations:
- AMZEEQ is a topical foam. While systemic absorption of AMZEEQ is low, and serious adverse reactions were not seen in clinical studies, the following adverse reactions associated with oral minocycline should be considered: Clostridioides difficile associated diarrhea, hepatotoxicity, metabolic effects, central nervous system effects, intracranial hypertension, autoimmune syndromes, photosensitivity, hypersensitivity reactions and tissue hyperpigmentation. Discontinue AMZEEQ immediately if serious adverse reactions occur with AMZEEQ.
- Patients should minimize or avoid exposure to natural or artificial sunlight while using AMZEEQ. Advise patients to discontinue treatment with AMZEEQ at the first evidence of sunburn.



